Pfizer (PFE) is an American multinational pharmaceutical company. Its major therapeutic focus areas are oncology, inflammation, rare disease, and vaccines. Pfizer teamed up with BioNTech (BNTX) to develop a COVID-19 vaccine that it sells under the Comirnaty brand.
Claim 50% Off TipRanks Premium
- Unlock hedge fund-level data and powerful investing tools for smarter, sharper decisions
- Stay ahead of the market with the latest news and analysis and maximize your portfolio's potential
For Q4 2021, Pfizer reported a 105% year-over-year jump in revenue to $23.8 billion but missed the consensus estimate of $24.2 billion. It posted adjusted EPS of $1.08, which rose from $0.43 in the same quarter the previous year and beat the consensus estimate of $0.88.
The company plans to distribute a quarterly dividend of $0.40 per share on March 4. Pfizer stock currently offers a dividend yield of 3.28%, compared to the sector average of 1.37%.
With this in mind, we used TipRanks to take a look at the newly added risk factors for Pfizer.
Risk Factors
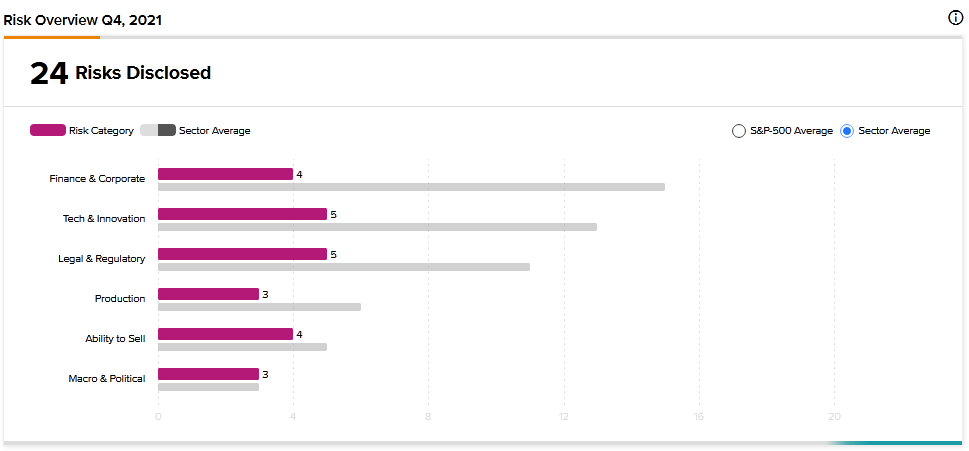
According to the new TipRanks Risk Factors tool, Pfizer’s main risk categories are Tech and Innovation and Legal and Regulatory, each with 5 of the total 24 risks identified for the stock. Finance and Corporate and Ability to Sell are the next two major risk categories with 4 risks each. The company has recently updated its profile with two new risk factors.
The company informs investors that the U.S. healthcare industry is not only highly regulated, but the regulations can also change frequently. Pfizer explains that efforts to reform the U.S. healthcare system, either by changing how healthcare is funded or provided, could have a significant impact on its business.
For example, the company mentions that a reduction in spending on programs such as Medicare and Medicaid may affect payments for its products and adversely impact its operating results. Further, Pfizer warns that the importation of competing prescription drugs into the U.S. could adversely impact its business.
In another newly added risk factor, Pfizer discusses the challenge of obtaining and maintaining regulatory approval for pharmaceutical products. It explains that the FDA may require additional studies to grant marketing authorization or approval of its products. For example, the FDA requires Pfizer to complete certain post-marketing studies for its COVID-19 vaccine Comirnaty by 2024.
Additionally, the company is required to complete certain studies for Paxlovid in relation to the FDA’s potential approval of the drug. Pfizer warns that additional studies could generate results that lead to the loss of marketing approval or changes in product labeling.
Analysts’ Take
Morgan Stanley analyst Matthew Harrison recently maintained a Hold rating on Pfizer stock but lowered the price target to $55 from $60. Harrison’s reduced price target still suggests 20.22% upside potential.
Consensus among analysts is a Moderate Buy based on 8 Buys and 9 Holds. The average Pfizer price target of $60.81 implies 32.92% upside potential to current levels.

Download the TipRanks mobile app now.
To find good ideas for stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.
Read full Disclaimer & Disclosure
Related News:
Report: Rivian Raises Vehicle Prices by up to 20%; Shares Sink 8.4%
Sea Dips 13.1% on Wider-Than-Expected Q4 Loss
Halliburton Opens Inaugural Oilfield Specialty Chemical Plant in Saudi Arabia
















