Shares of pharmaceutical company Bristol Myers Squibb (BMY) are up 4% on news that the U.S. Food and Drug Administration (FDA) has approved its new schizophrenia drug called “Cobenfy.”
Don't Miss our Black Friday Offers:
- Unlock your investing potential with TipRanks Premium - Now At 40% OFF!
- Make smarter investments with weekly expert stock picks from the Smart Investor Newsletter
The medication is the first new treatment for the mental health condition in seven decades. Following the FDA approval, Bristol Myers Squibb said that Cobenfy, which is a pill taken twice daily, will be widely available in the U.S. by late October.
The medication is priced at $1,850 for a month’s supply or $22,500 annually. However, Bristol Myers Squibb said the prescription drug should be covered by most U.S. health insurance plans. Schizophrenia is a mental health condition that causes auditory hallucinations in people. An estimated three million adults in the U.S. live with the disease.
A Potential Blockbuster Drug
Analysts say that Cobenfy could provide Bristol Myers Squibb with strong sales and might become a blockbuster medication for the pharmaceutical concern. In a recent research note, analysts at brokerage Guggenheim said that Cobenfy is a “multi-billion-dollar opportunity” for Bristol Myers Squibb.
Bristol Myers Squibb acquired the drug Cobenfy through its $14 billion purchase of biotech company Karuna Therapeutics last year. The boost provided to BMY stock from the FDA approval of Cobenfy is no doubt welcome news for the company’s shareholders. In the past year, the share price of Bristol Myers Squibb has declined 8%.
Is BMY Stock a Buy?
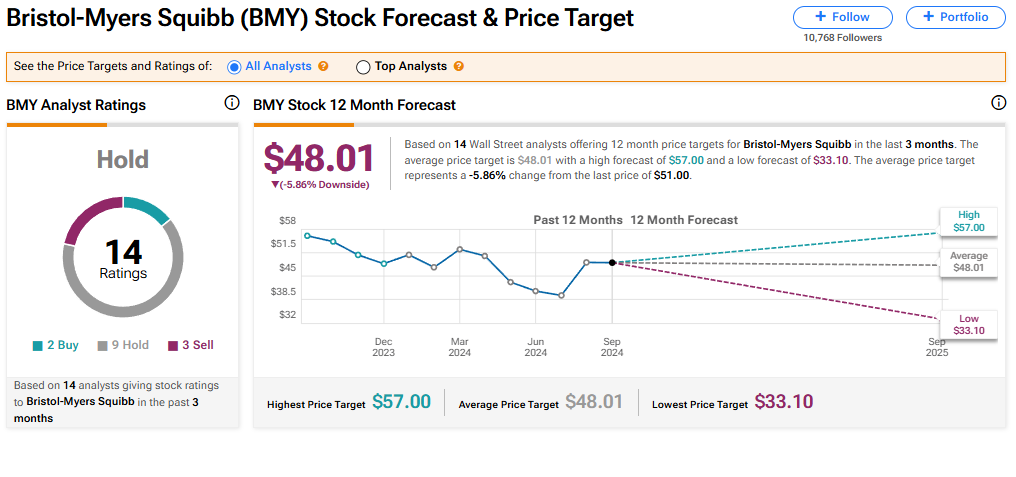
The stock of Bristol Myers Squibb has a consensus Hold rating among 14 Wall Street analysts. That rating is based on two Buy, nine Hold and three Sell recommendations made in the last three months. The average price target on BMY stock of $48.01 implies 5.86% downside from current levels.