Patient enrollment has accelerated, now with 13 patients enrolled in trial
TFF TAC at ~20% of the oral tacrolimus dose prevented acute rejection and achieved >80% of the previous oral trough blood levels — leading to diminished drug burden
9 out of 9 (100%) patients who completed the 12-week treatment chose to remain on TFF TAC by proceeding to the long-term extension Phase; 2 patients have been treated for over a year and 6 patients have been treated for more than 6 months
Observed a 6.5-fold reduction in the number of abnormally expressed rejection-related gene sets after treatment with TFF TAC
No production of donor-specific antibodies (DSA) after treatment with TFF TAC
FORT WORTH, Texas, Aug. 06, 2024 (GLOBE NEWSWIRE) — TFF Pharmaceuticals, Inc. (NASDAQ: TFFP) (“the Company”), a clinical-stage biopharmaceutical company focused on developing and commercializing innovative drug products based on its patented Thin Film Freezing (TFF) technology platform, today provided an update from the Company’s ongoing Phase 2 trial of Tacrolimus Inhalation Powder (TFF TAC) for the prevention of lung transplant rejection.
“The growing body of data from the TFF TAC Phase 2 study continues to suggest that TFF TAC has transformative potential to advance the field of immunosuppressive therapy to prevent lung transplant rejection,” said Dr. Harlan Weisman, Chief Executive Officer of TFF Pharmaceuticals. “We are finalizing the design of the next study with TFF TAC in close collaboration with our clinical investigators and in communication with regulatory authorities and look forward to providing additional updates on the program including a regulatory update later in the fall.”
Clinical Trial Update
- Patient enrollment has accelerated with 13 patients now enrolled in Phase 2 trial
- TFF TAC at ~20% of the oral tacrolimus dose prevented acute rejection and achieved >80% of the oral trough blood levels leading to diminished drug burden
- No signs or symptoms suggestive of acute rejection
- No use of pulse corticosteroids for treatment of rejection
- No spirometry deterioration suggestive of acute rejection
- No chest x-ray findings suggestive of acute rejection
- No biomarker evidence of acute rejection (gene expression and donor-specific antibody)
- Lower doses and no first pass effect to generate drug metabolites decreases drug burden with TFF TAC compared with oral tacrolimus.
- 9 out of 9 (100%) patients who completed the 12-week treatment period with TFF TAC chose to remain on the therapy by proceeding to the long-term extension phase;
- 2 patients have been treated for over 1 year, and 6 patients have been treated for more than 6 months;
- Total patient exposure to TFF TAC therapy has now reached 2,063 days, or a total of 5.65 years.
- PK data from the Phase 2 study continue to indicate that TFF TAC dosing results in reduced systemic variability of tacrolimus; the systemic tacrolimus trough to peak concentration swings that occur with oral tacrolimus are not present with TFF TAC, which is predicted to reduce the risk of organ rejection and systemic toxicities such as chronic kidney disease.
- Confirmatory biomarker data from the Phase 2 study also remain positive:
- Updated biomarker data indicate a 6.5-fold reduction in the number of abnormally expressed rejection-related gene sets after 12 weeks of treatment with TFF TAC. These data further suggest TFF TAC has the potential to provide sufficient immunosuppression to prevent rejection
- 23% to 4% reduction (-85%) in the expression of rejection related genes:
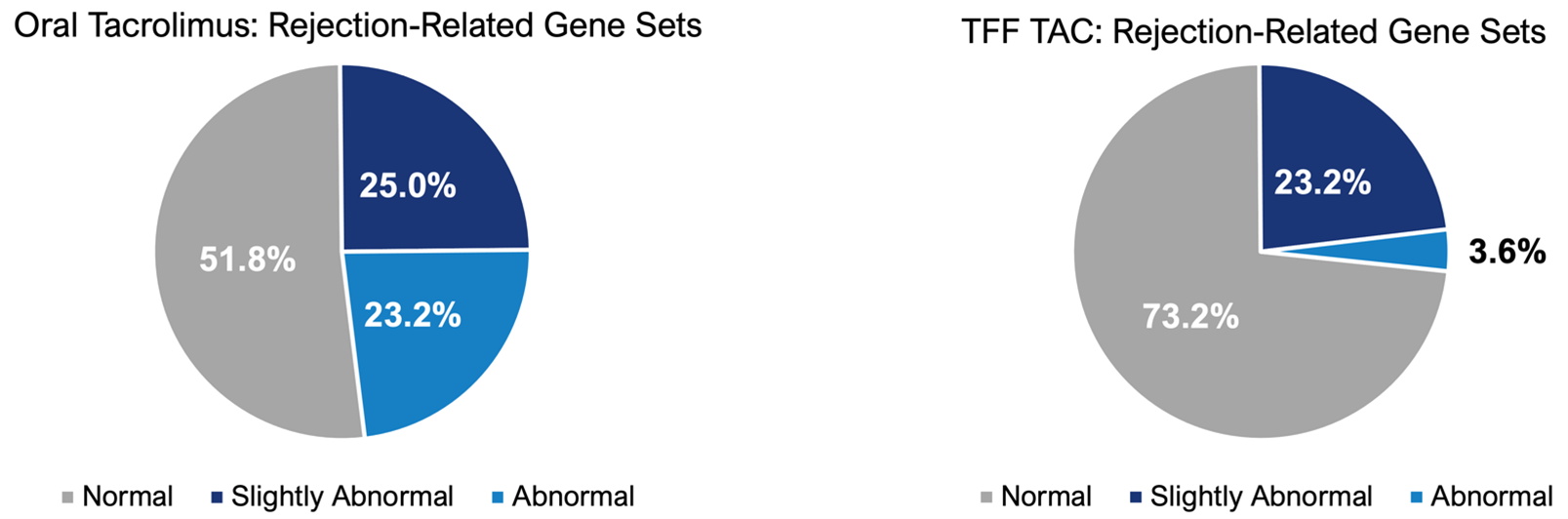
- 23% to 4% reduction (-85%) in the expression of rejection related genes:
- New biomarker data exploring the presence of donor-specific antibodies (DSA) are now available in the first 8 patients from the study. DSA is known to drive antibody-mediated rejection and is generated when there is insufficient immune suppression systemically allowing the formation of antibodies in the lymph nodes against the transplanted (donor) organ. DSA was negative for the first 8 patients on oral tacrolimus, and DSA remained negative after 12 weeks of treatment with TFF TAC.
- Updated biomarker data indicate a 6.5-fold reduction in the number of abnormally expressed rejection-related gene sets after 12 weeks of treatment with TFF TAC. These data further suggest TFF TAC has the potential to provide sufficient immunosuppression to prevent rejection
- With respect to TFF TAC safety and tolerability, there has been no mortality. The majority of treatment emergent adverse events were Grade 2 (moderate) or lower with no bronchospasm or wheezing reported. Kidney function has been maintained.
- One patient was transitioned to a dose of TFF TAC that was too low, which led to blood trough levels that were >50% below the protocol-specified minimum. This patient experienced signs and symptoms of acute rejection (but minimal on histopathology). TFF TAC was discontinued as required by the protocol and oral tacrolimus was resumed. The acute rejection episode has resolved.
- TFF is finalizing the design of the next study with TFF TAC in close collaboration with clinical investigators and in communication with regulatory authorities and plans to provide additional updates on the program including a regulatory update later in the fall.
“As the number of patients treated in our Phase 2 study continues to grow, we are highly encouraged by the emerging positive product profile for TFF TAC,” said Dr. Zamaneh Mikhak, Chief Medical Officer of TFF Pharmaceuticals. “For this update, we showed the available new data related to DSA for the first eight patients in the study. This analysis indicates that no patients have developed DSA after transitioning from oral tacrolimus to TFF TAC, suggesting sufficient systemic immune suppression to inhibit production of potentially harmful antibodies despite the modest decrease in blood trough levels. In addition, our updated gene expression data from now 8 patients show a dramatic 6.5-fold reduction in the number of abnormally expressed rejection-related gene sets, suggesting that TFF TAC has the potential to impart sufficient levels of immunosuppression to prevent rejection.”
ABOUT TFF PHARMACEUTICALS’ THIN FILM FREEZING (TFF) TECHNOLOGY
TFF Pharmaceuticals’ proprietary Thin Film Freezing (TFF) technology allows for the transformation of both existing compounds and new chemical entities into dry powder formulations exhibiting unique characteristics and benefits. The TFF process is a particle engineering process designed to generate dry powder particles with advantageous properties for inhalation, as well as parenteral, nasal, oral, topical and ocular routes of administration. The process can be used to engineer powders for direct delivery to the site of need, circumventing challenges of systemic administration and leading to improved bioavailability, faster onset of action, and improved safety and efficacy. The ability to deliver therapies directly to the target organ, such as the lung, allows TFF powders to be administered at lower doses compared to oral drugs, reducing unwanted toxicities and side effects. Laboratory data suggests the aerodynamic properties of the powders created by TFF can deliver as much as 75% of the dose to the deep lung. TFF does not introduce heat, shear stress, or other forces that can damage more complex therapeutic components, such as fragile biologics, and instead enables the reformulation of these materials into easily stored and temperature-stable dry powders, making therapeutics and vaccines more accessible for distribution worldwide. The advantages of TFF can be used to enhance traditional delivery or combined to enable next-generation pharmaceutical products.
ABOUT TFF PHARMACEUTICALS
TFF Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company engaging patented rapid freezing technology to develop and transform medicines into potent dry powder formulations for better efficacy, safety, and stability. The company’s versatile TFF technology platform has broad applicability to convert most any drug, including vaccines, small and large molecules, and biologics, into an elegant dry powder highly advantageous for inhalation or for topical delivery to the eyes, nose and skin.
SAFE HARBOR
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements in this press release include, but are not limited to, statements by the Company relating to the innovation and commercial potential of the Company’s TFF TAC product candidate to advance the field of immunosuppressive therapy to prevent lung transplant rejection. Forward-looking statements involve known and unknown risks, uncertainties and other factors that could cause actual results to differ materially, including (i) the risk that further data from the Company’s ongoing Phase 2 trial of TFF TAC may not be consistent with the positive preliminary data obtained to date, (ii) the risk that the Company may not be able to obtain additional working capital with which to continue its current operations and clinical trials as and when needed, (iii) success in early phases of pre-clinical and clinical trials do not ensure later clinical trials will be successful; (iv) no drug product incorporating the TFF platform has received FDA pre-market approval or otherwise been incorporated into a commercial drug product, (v) the Company has no current agreements or understandings with any large pharmaceutical companies for the development of a drug product incorporating the TFF Platform, and (vi) those other risks disclosed in the section “Risk Factors” included in the Company’s Quarterly Report on Form 10-Q filed with the SEC on May 14, 2024. The Company cautions readers not to place undue reliance on any forward-looking statements. The Company does not undertake and specifically disclaims any obligation to update or revise such statements to reflect new circumstances or unanticipated events as they occur, except as required by law.
Investor Relations Contact:
Corey Davis, Ph.D.
LifeSci Advisors
(212) 915-2577
cdavis@lifesciadvisors.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/69052a47-d65e-4cb7-9528-92212f06cb89



















