Global pharmaceutical company Merck (NYSE: MRK) announced that it has received a positive Committee for Medicinal Products for Human Use (CHMP) opinion from the European Medicines Agency (EMA) for KEYTRUDA, an anti-programmed death receptor-1 (PD-1) therapy.
Claim 50% Off TipRanks Premium
- Unlock hedge fund-level data and powerful investing tools for smarter, sharper decisions
- Stay ahead of the market with the latest news and analysis and maximize your portfolio's potential
KEYTRUDA is the first-of-its-kind anti-PD-1 therapy to treat various kinds of microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) tumors in adults. The injection increases the body’s immune system to help detect and fight tumor cells.
Supporting Data
Merck said that the CHMP’s recommendation followed results from both Phase 2 KEYNOTE-158 trial and Phase 2 KEYNOTE-164 trial. Remarkably, the U.S. Food and Drug Administration also granted accelerated approval to KEYTRUDA based on the results from these trials. The therapy was approved as the first cancer treatment based on a biomarker in MSI-H or dMMR solid tumors.
The European Commission (EC), which approves medicines for the European Union (EU), will now review the CHMP recommendation and is expected to announce the final decision in the second quarter of 2022.
Official Comments
Merck’s Clinical Research VP Dr. Scot Ebbinghaus said, “This positive CHMP opinion reinforces the predictive value of MSI-H/dMMR across many different cancer types and the importance of biomarker testing.”
Another Milestone
Merck was also granted a positive CHMP opinion from the EMA for KEYTRUDA, along with chemotherapy, with or without bevacizumab (the KEYTRUDA regimen). It is expected to treat persistent, chronic, or metastatic cervical cancer in adults whose tumors express PD-L1.
The recommendation was based on the data from the Phase 3 KEYNOTE-826 trial, in which the KEYTRUDA regimen showed a statistically significant improvement in overall survival and progression-free survival compared to chemotherapy with or without bevacizumab.
Smart Score
Merck scores a “Perfect 10” on TipRanks’ Smart Score rating system, indicating that the stock has strong potential to outperform market expectations.
Wall Street’s Take
Recently, Wells Fargo analyst Mohit Bansal maintained a Buy rating on Merck and a price target of $90 (10.65% upside potential).
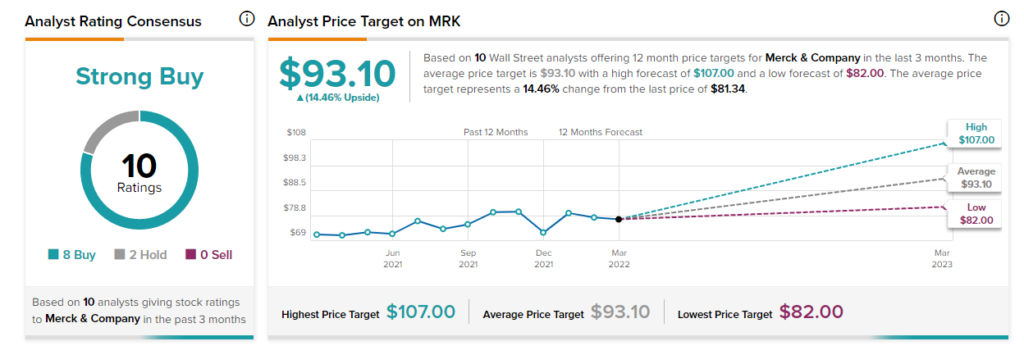
Shares of Merck have rallied 12.91% over the past year, while the stock still scores a Strong Buy consensus rating, based on eight Buys and two Holds. The average Merck price target stands at $93.10 and implies upside potential of 14.46% to current levels.
Download the TipRanks mobile app now
To find good ideas for stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.
Read full Disclaimer & Disclosure
Related News:
Pfizer Receives FDA Breakthrough Therapy Designation for RSVpreF
BP Hopes to Sell Shut-Down North Sea Oilfield
GameStop Jumps 14.5% on Cohen’s Stake Increase
















