Shares of biopharmaceutical company Synlogic (NASDAQ:SYBX) are on the rise today after its product candidate SYNB1934 bagged an orphan drug designation from the U.S. Food and Drug Administration for the treatment of phenylketonuria (PKU).
Meet Your ETF AI Analyst
- Discover how TipRanks' ETF AI Analyst can help you make smarter investment decisions
- Explore ETFs TipRanks' users love and see what insights the ETF AI Analyst reveals about the ones you follow.
Next, Synlogic is planning to begin a Phase 3 trial of the drug in the first half of 2023. Impressively, SYNB1934 has also bagged a Rare Pediatric Disease designation in the U.S. as well as an orphan drug designation in Europe.
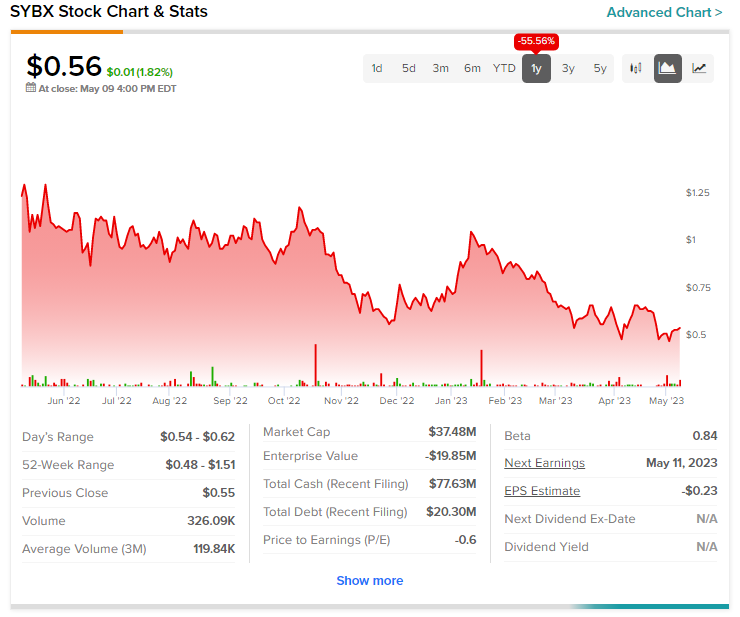
Today’s price gains come after a nearly 55.5% drop in Synlogic shares over the past year.
Read full Disclosure
















