Soleno Therapeutics (SLNO) stock jumped over 32% in yesterday’s after-hours trading. The surge came after the company announced that the U.S. Food and Drug Administration (FDA) had approved VYKAT XR, its new treatment for hyperphagia associated with Prader-Willi syndrome (PWS).
Confident Investing Starts Here:
- Easily unpack a company's performance with TipRanks' new KPI Data for smart investment decisions
- Receive undervalued, market resilient stocks right to your inbox with TipRanks' Smart Value Newsletter
PWS is a rare genetic disorder that affects about 50,000 people in the U.S. and often leads to excessive hunger, severe obesity, and other health complications. The approval of VYKAT XR offers a critical new option for managing the condition, addressing a major unmet medical need.
With VYKAT XR expected to hit the U.S. market in April 2025, Soleno is gearing up for its commercial rollout. The company has introduced Soleno One, a support program to help patients and caregivers get access to treatment.
Stock Jumps as Investors Bet on Future Growth
Trading was briefly halted pending the FDA approval, but once resumed, Soleno’s stock soared from $42.80 to $65, reflecting growing investor confidence.
The approval could pave the way for Soleno to generate revenue in the future. In its latest Q4 earnings report, the company reported $318.6 million in cash and cash equivalents, which will help fund its commercial launch. Market observers expect sales of VYKAT XR to contribute meaningfully to Soleno’s revenue in 2025 and beyond, though long-term demand remains to be seen.
Is Soleno Therapeutics a Good Buy?
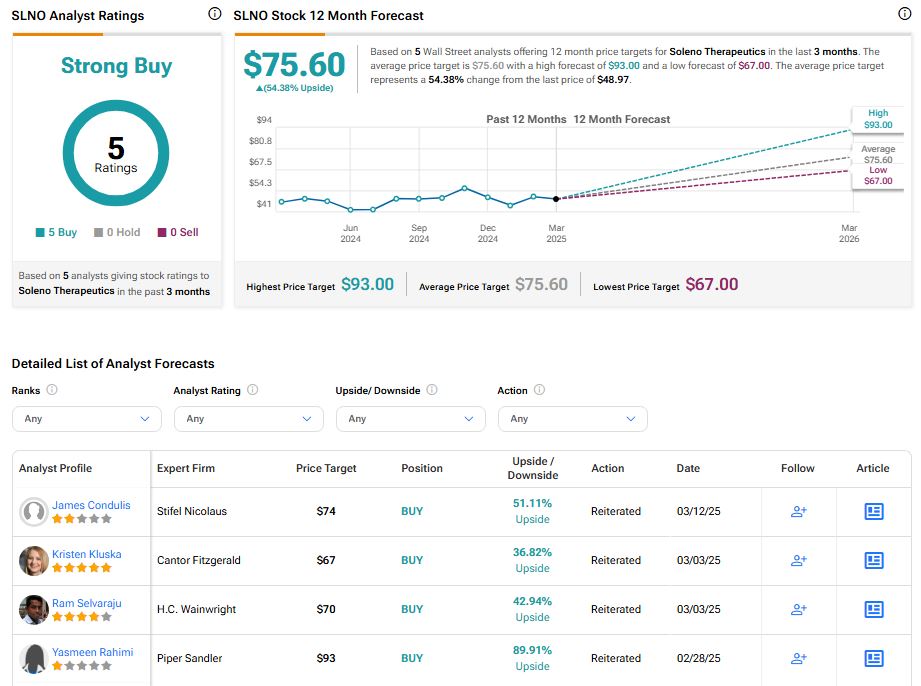
Wall Street is optimistic on Soleno Therapeutics stock, with a Strong Buy consensus rating based on unanimous five Buys. The average SLNO stock price target of $75.60 implies an upside risk of 54.38% from current levels.
Looking for a trading platform? Check out TipRanks' Best Online Brokers guide, and find the ideal broker for your trades.
Report an Issue















