Shares of Pharvaris (NASDAQ: PHVS) soared by more than 60% in morning trading on Thursday after the clinical-stage company announced positive top-line data for PHVS416. RAPIDe-1 is a Phase 2 study of PHVS416 used for the on-demand treatment of hereditary angioedema (HAE) attacks.
Claim 50% Off TipRanks Premium
- Unlock hedge fund-level data and powerful investing tools for smarter, sharper decisions
- Stay ahead of the market with the latest news and analysis and maximize your portfolio's potential
This Phase-2 study demonstrated “statistically significant results of PHVS416 as an oral on-demand treatment for HAE attacks.”
Berndt Modig, CEO of Pharvaris commented, “The results of the RAPIDe-1 study affirm our confidence in our clinical development program in HAE. Top-line data from CHAPTER-1, a proof-of-concept study of PHVS416 for the prophylactic treatment of HAE, is anticipated in the second half of 2023.”
The company also announced its Q3 results with a loss of €0.25 per share, compared to €0.28 in the same period last year. At the end of Q3, PHVS had cash and cash equivalents of €198 million.
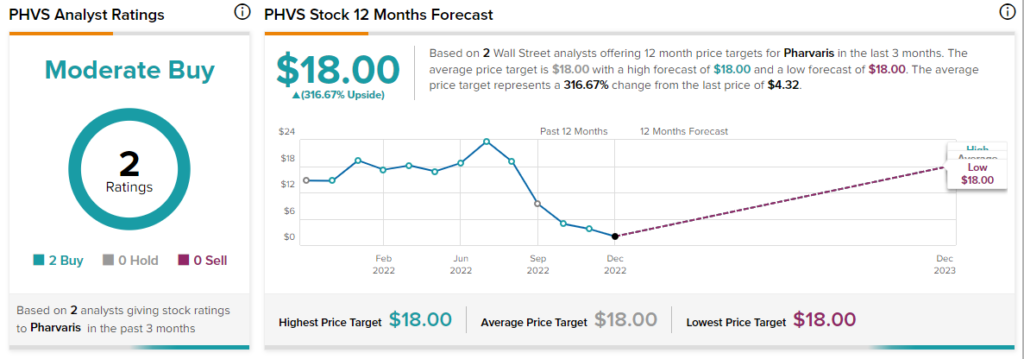
Only two analysts have covered the stock in the past three months with both of them rating PHVS stock a Buy.
















