Morphic Therapeutic (NASDAQ: MORF), a biopharmaceutical company jumped in morning trading on Tuesday after the company reported positive topline data from the open-label EMERALD-1 Phase 2a study of MORF-057, an oral small molecule inhibitor of the α4β7 integrin, in adults with moderate to severe ulcerative colitis (UC), a form of chronic inflammatory bowel disease (IBD).
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
The Phase 2a study of MORF-057 indicated that MORF-057 met the “primary endpoint and demonstrates clinically meaningful improvements across secondary and exploratory measures with no safety signal observed.”
In addition, Morphic also announced its fiscal Q1 results with a net loss of $0.9 per share versus a net loss of $0.85 per share in the same period last year. This loss was wider than analysts’ expectations of a loss of $0.81 per share. The company posted revenues of $0.5 million in the first quarter versus $2.4 million in the same period last year which was again lower than Street estimates of $2.16 million.
Praveen Tipirneni, MD, CEO of Morphic Therapeutic commented, “Q1 was a pivotal quarter for Morphic, with the EMERALD-1 study reporting positive topline results, including clinically meaningful activity and favorable tolerability. In addition, we extended our cash runway into the second half of 2026, well beyond the primary endpoint readout of EMERALD-2, the phase 2b randomized study of MORF-057 in UC, in the first half of 2025.”
As of March 31, 2023, Morphic had cash, cash equivalents and marketable securities of $421 million, compared to $348 million at the end of the fourth quarter, with the “increase primarily due to a private placement of Morphic’s common stock and pre-funded warrants with certain existing investors that was completed in February 2023.”
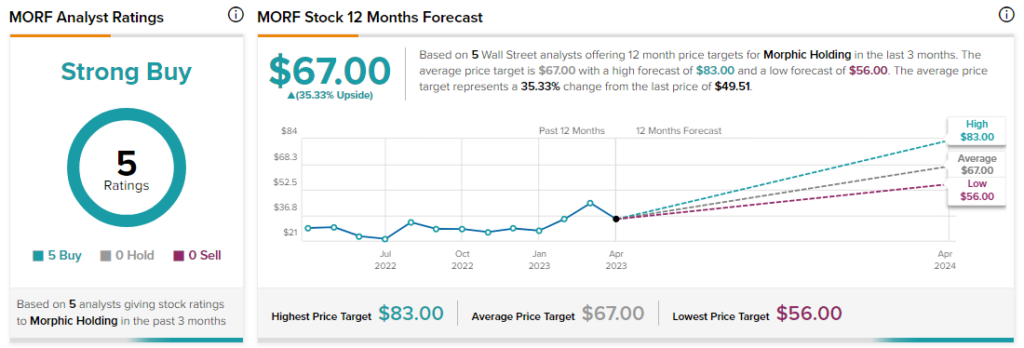
Wall Street analysts remain bullish about MORF stock with a Strong Buy consensus rating based on five Buys.
















