Shares of a clinical-stage biotechnology company, Larimar Therapeutics, (NASDAQ: LRMR) crashed in morning trading at the time of publishing on Monday even as the company announced positive topline data from the 25 mg cohort of its Phase 2, placebo-controlled, dose exploration trial of CTI-1601 in participants with Friedreich’s ataxia (FA) with a duration of four weeks.
Claim 50% Off TipRanks Premium
- Unlock hedge fund-level data and powerful investing tools for smarter, sharper decisions
- Stay ahead of the market with the latest news and analysis and maximize your portfolio's potential
This trial indicated that CTI-1601 was well-tolerated when administered daily for 14 days and then every other day thereafter until day 28. This trial also indicated that daily subcutaneous injections of CTI-1601 showed “increases in frataxin levels from baseline compared to placebo in all evaluated tissues.”
The company has submitted the results of this trial to the U.S. FDA and an update is expected later this year.
In addition, Larimar also announced its fiscal Q1 results as losses narrowed to $0.15 per share versus $0.49 per share in the same period last year. As of March 31, the company had cash and cash equivalents totaling $111.5 million.
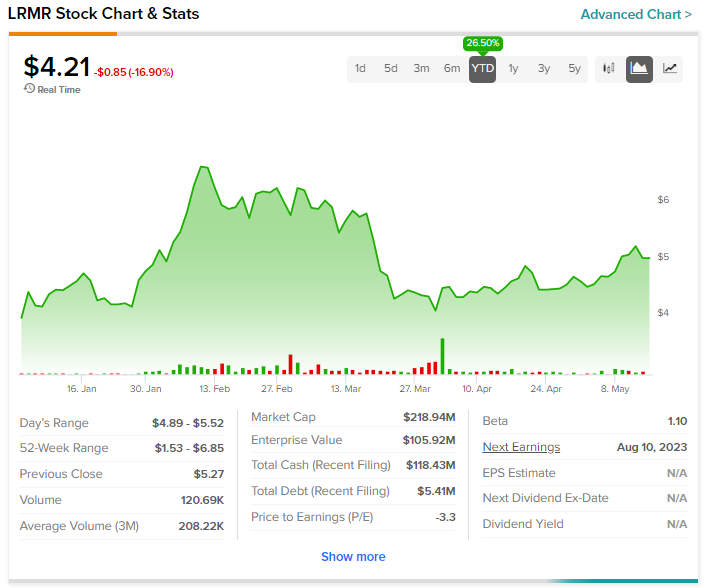
LRMR stock has soared by more than 25% year-to-date.
















