Shares of biopharmaceutical company Immuron Ltd. (NASDAQ:IMRN) are soaring higher today after the U.S. Food and Drug Administration approved its Travelan IND (investigational new drug) application.
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
The IND application will evaluate the drug’s efficacy at a single dose for the treatment of infectious diarrhea resulting from ETEC (Escherichia coli). Consequently, the company now plans to begin recruitment for the trial in H1 2023.
Headline results from the study are anticipated by the end of 2023. Infectious diarrhea is characterized by one of the highest prevalence rates in travelers to developing geographies and among U.S. defense forces deployed worldwide.
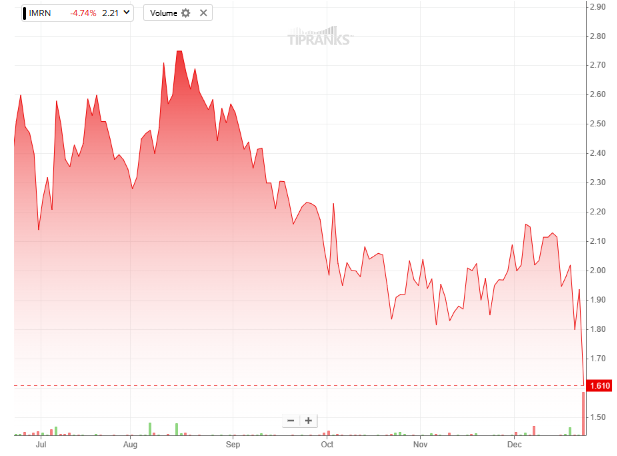
Shares of the company have dropped nearly 32% over the past six months.
Read full Disclosure
















