Shares of biopharmaceutical company Immuron (NASDAQ:IMRN) are soaring higher at the time of publishing today after the U.S. Food and Drug Administration (FDA) lifted a hold on the investigational new drug application for an oral therapeutic targeted at Campylobacter and enterotoxigenic Escherichia coli (ETEC).
Elevate Your Investing Strategy:
- Take advantage of TipRanks Premium at 50% off! Unlock powerful investing tools, advanced data, and expert analyst insights to help you invest with confidence.
With this development, the company can now evaluate the efficacy of the product candidate in preventing infectious diarrhea caused by Campylobacter and ETEC.
The product will now be evaluated in two trials with 60 subjects. While antibiotic prescription has been the first line of treatment for infectious diarrhea, pathogens have displayed increasing resistance to common prescriptions over the past few years.
Consequently, preventive treatment for the condition is now gaining priority in the medical field as well as defense establishments.
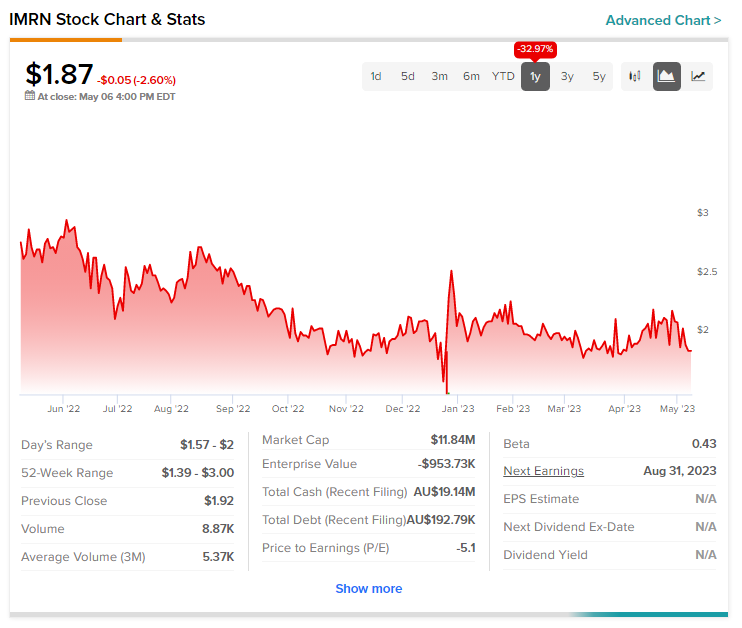
Today’s price rise comes after a nearly 33% slide in Immuron shares over the past year.
Read full Disclosure
















