With top-and-bottom-line beats for the most recent quarter, Arcturus Therapeutics (ARCT) is making progress in unleashing the power of mRNA to revolutionize treatments for infectious diseases, liver issues, and rare respiratory diseases. The company has a number of promising ongoing clinical trials progressing, having recently received clearance from the FDA for a Phase 2 study for ARCT-032 and for ARCT-810, an mRNA therapeutic for OTC deficiency.
Arcturus also announced impressive post-vaccination data for KOSTAIVE, with superior results against key variants, even at one-sixth the dose of conventional vaccines. Adding Japanese manufacturing sites for KOSTAIVE will enable domestic production as the company continues to progress in commercializing the treatment. It appears to be an attractive option for investors interested in a promising biotech.
Arcturus Progressing with Promising Pipeline
Arcturus Therapeutics is a biopharmaceutical company specializing in mRNA medicines and vaccines for infectious and rare liver and respiratory diseases. Its technology revolves around LUNAR lipid-mediated delivery and STARR mRNA.
The company is progressing with its clinical pipeline. It recently obtained FDA clearance for an Investigational New Drug application, enabling the initiation of a Phase 2 study for ARCT-032, a potential treatment for cystic fibrosis (CF). Interim data from this Phase 2 study are expected in the first half of 2025.
Furthermore, Arcturus expanded its Phase 2 clinical program of ARCT-810, an mRNA therapeutic for ornithine transcarbamylase (OTC) deficiency. A multiple-dose study currently enrolls adults and adolescents requiring clinical management for this deficiency. Interim data from the Phase 2 trials in the U.S. and Europe are on track to be released in the first half of 2025.
Finally, the company introduced new 12-month post vaccination data for KOSTAIVE showing superior immunogenicity compared to the conventional mRNA vaccine COMIRNATY® against several variants, even at a lesser dosage.
Analysis of Arcturus Recent Financial Results
The company recently posted results for the third quarter. Revenue of $41.67 million beat analysts’ expectations by $5.08 million. Yet, it was slightly down from the corresponding period last year, mainly due to a decrease in milestone achievements from the CSL agreement. However, this was counterbalanced by revenue from a supply agreement and increased revenue from the BARDA agreement.
Operating expenses dipped from $64.5 million to $52.4 million. Research and development expenses also decreased, primarily due to lower manufacturing costs for the COVID program. General and administrative expenses remained consistent year-over-year. Arcturus posted a net loss of $6.9M or -$0.26 per diluted share, which surpassed consensus expectations by $0.96.
As of the quarter’s end, the company had cash and cash equivalents of $294.1 million, with a cash runway expected to last through the first quarter of 2027.
Is ARCT Stock a Buy?
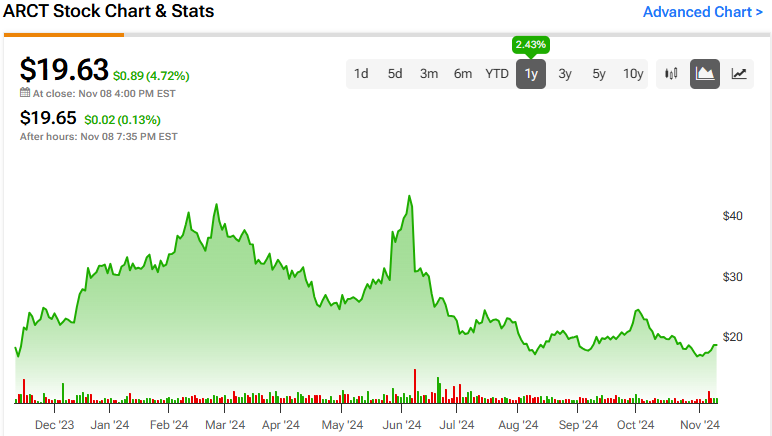
The stock has been highly volatile, with a beta of 2.39, bouncing around over the past year, earning a 2.43% return. It trades at the lower end of its 52-week price range of $17.26 – $45.00. The P/S ratio of 3.6x sits below the Biotechnology industry average of 9.3x, suggesting the stock trades at a relative discount.
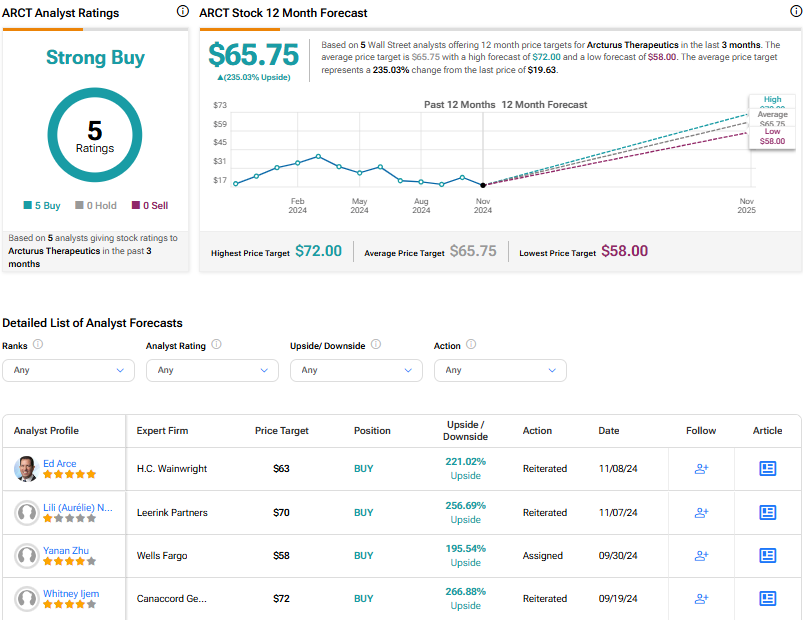
Analysts following the company have been bullish on ARCT stock. Based on 5 analysts’ recent recommendations, Arcturus Therapeutics is rated a Strong Buy overall. The average price target for ARCT stock is $64.33, which represents a potential upside of 227.80% from current levels.
Final Analysis on ARCT
Arcturus Therapeutics has shown significant potential with its progress in clinical trials, including ARCT-032 for cystic fibrosis and ARCT-810 for OTC deficiency. The groundbreaking KOSTAIVE vaccine has demonstrated superior results at a fraction of the standard dose. With a robust cash runway and a portfolio of promising therapeutics, Arcturus is well-positioned for future success. The company’s stock, trading at a relative discount, offers a compelling opportunity for investors eager to capitalize on the burgeoning biotech sector.
















