A class action lawsuit was filed against Agenus Inc. (AGEN) by Levi & Korsinsky on September 6, 2024. The plaintiffs (shareholders) alleged that they bought AGEN stock at artificially inflated prices between January 23, 2023 and July 17, 2024 (Class Period) and are now seeking compensation for their financial losses. Investors who bought Agenus stock during that period can click here to learn about joining the lawsuit.
Confident Investing Starts Here:
- Easily unpack a company's performance with TipRanks' new KPI Data for smart investment decisions
- Receive undervalued, market resilient stocks right to your inbox with TipRanks' Smart Value Newsletter
Agenus is a clinical-stage biotechnology company focused on developing innovative treatments for people suffering from cancer. Agenus is an inmuno-oncology company engaged in leveraging the body’s immune system to fight cancer and infectious diseases.
Agenus’ claims about the development of “botensilimab/balstilimab combination drug,” an investigational therapy for the treatment of patients with metastatic colorectal cancer (CRC), are at the heart of the current complaint.
Agenus’ Misleading Claims
According to the lawsuit, Agenus and three of its senior officers (Individual Defendants) repeatedly made false and misleading public statements throughout the Class Period. Particularly, they are accused of omitting truthful information about the efficacy of the botensilimab (BOT)/balstilimab (BAL) combination and ancillary issues from SEC filings and related material.
For instance, during the Class Period, the defendants time and again reiterated the importance of the combined power of BOT and BAL, on a stand-alone or combined basis, in the treatment of a broad spectrum of cancer.
In a March 2023 press release, the CEO stated that Agenus has entered 2023 with a solid and diverse clinical pipeline of immune-oncology programs. Similarly, in an October 2023 press release, the company’s CMO stated that BOT alone or in combination with BAL or other standard therapies positions Agenus to provide effective care for cancer patients in early and late-stage tumors.
However, subsequent events (discussed below) revealed that Agenus allegedly misled investors about the potential benefits of the combined therapeutic power of BOT and BAL and the company’s future prospects.
Plaintiffs’ Arguments
The plaintiffs maintain that the Defendants deceived investors by lying and withholding critical information about the company’s business practices and prospects during the Class Period. Importantly, the Defendants are accused of misleading investors about the efficacy of the combination of BOT and BAL in the treatment of cancer.
The information became clear in a press release issued on July 18, 2024. The company announced the results from the end of Phase 2 (EOP2) meeting with the U.S. FDA (Food and Drug Administration) for the prospective future combination of “botensilimab (BOT) and balstilimab (BAL) for the treatment of adult patients with relapsed/refractory microsatellite stable colorectal cancer (r/rMSS CRC) with no active liver metastases (NLM).”
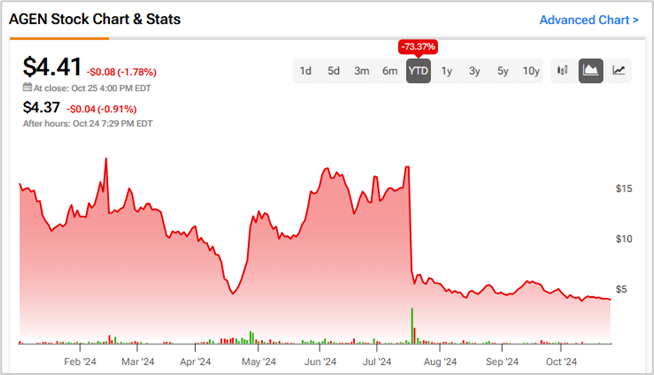
In this context, the FDA noted that it is not advisable to submit the results in support of an accelerated approval of the combined drug. This is because the FDA believed that “objective response rates may not translate to survival benefit.” Following the news, AGEN shares collapsed 58.8% on July 18.
To conclude, Agenus made unrealistic claims about the potential of the combined power of botensilimab (BOT) and balstilimab (BAL) in the treatment of certain cancers and the commercialization prospects. Year-to-date, AGEN stock has lost 73.4%, causing massive damage to shareholder returns.
Looking for a trading platform? Check out TipRanks' Best Online Brokers , and find the ideal broker for your trades.
Report an Issue









